Photographic Film
Background
Photographic film is a chemically reactive material that records a fixed or still image when the film is exposed to light. Typically, film is placed in a camera, and light from the image being photographed is allowed to enter and is focused and sometimes made larger or smaller by the camera lens. The film is exposed to the image by opening a shutter in the camera body, and the combination of the speed of the shutter and the film speed (which is the chemical reactivity of the film) controls the amount of light that strikes the film. The image is recorded on the film, but it is a latent or invisible image. When the film is removed from the camera, it is developed by chemical processes into a visible image. This visible image is negative or the reverse in brightness of the way our eyes see light; the brightest parts of the photographed object appear the darkest on the negative where the film received the most exposure to light. The negative image is made positive, or as our eyes see it, by another type of processing whereby the negative is printed on sensitive paper. Color-reversal films are positives and are used for making slides. All of the elements of the process—the parts of the camera, the type and parts of the lens, the type of film, including its chemistry, the developing process, the printing process, and the type of paper—contribute to the sharpness or trueness of the finished photograph.
History
Film was "discovered" in a chemistry laboratory. In 1727, Johann Henrich Schulze, a German doctor, mixed chalk, silver, and nitric acid in a flask to make silver nitrate. When the solution was exposed to sunlight, it changed color from white to purple. When Schulze pasted cutouts of letters and numbers on the outside of a flask of freshly made solution and exposed it to the light, the cutouts appeared to have been printed on the solution. Although the discovery marked the birth of photography, it was not used for over 100 years. In 1839, Louis Daguerre, a French painter, created a photographic process in which liquid iodine was placed on a silvered copper plate, and the plate was exposed to light. The liquid iodine was the emulsion, or light-reactive chemical, and the copper plate was the base for these photographs called "daguerreotypes." The American inventor Samuel F.B. Morse learned the art of daguerreotypy and taught it to Matthew Brady, who made images of the Civil War that are treasured both as historical records and artistic landmarks in photography.
Daguerreotypy was cumbersome to use; the "wet plate" process was awkward, the box-type cameras had to hold the large plates, and the finished photographs were the size of the plates. While Daguerre was developing his process, William Henry Fox Talbot, an English archaeologist, created his own process called "calotype," meaning "beautiful picture" in 1841. Talbot coated a paper base with an emulsion of silver iodide and produced a negative by a developing process. The calotype is more like today's film and photographic process, and the intermediate step resulting in a negative permitted more than one print to be made.
The flexibility of photography was improved further in 1871 when R.L. Maddox invented the "dry plate" process. Gelatin
George Eastman combined the paper base of Talbot's calotype with the gelatinous silver nitrate emulsion from Maddox's process to invent flexible roll film in 1884. Eastman quickly made the transition to an emulsion-bearing plastic, transparent film by 1889, which was a year after his company introduced the first Kodak camera. These developments made photography a simple, compact, portable practice that is now the most popular hobby in the United States.
Raw Materials
A roll of film consists of the emulsion and base that compose the film itself, the cassette or cartridge, and outer protective packaging. The materials used to make the emulsion are silver, nitric acid, and gelatin. The base consists of cellulose and solvents that are mixed to form a thick fluid called dope. Film that is packed in a cassette (35-millimeter film is typically packed this way) requires a metal spool, the protective metal canister, and plastic strips at the canister opening where the film emerges. Other sizes of film including Polaroid film are protected from light and air by plastic cartridges or packs. Outer packaging, which varies among film products, is made from foil-lined paper, plastic, and thin cardboard cartons. The outer packaging is also insulating and protects the film from exposure to light, heat, and air.
The Manufacturing
Process
Base
-
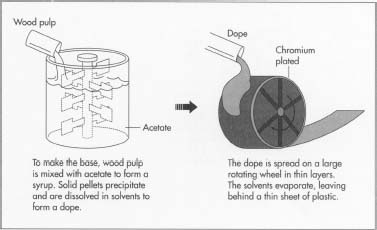
1 For most films, the base to which the light-sensitive emulsion is
fixed consists of cellulose acetate, which is wood pulp or cotton
linters (short cottonseed fibers) mixed with acetate to form a syrup.
Solid pellets of cellulose acetate precipitate or separate out of the
syrup and are washed and dried. The pellets are dissolved in solvents to
form the transparent, honey-like dope. The dope is spread in a thin,
even sheet on a wheel that is two stories in diameter. The wheel is
plated with chromium for a smooth finish, and it turns slowly. The
solvents in the dope volatilize or evaporate as the wheel turns. The
process is much like the applying and drying of nail polish. The
remaining base is a thin sheet of plastic that is of a uniform thickness
measured in ten-thousandths of an inch. When it is dry, the base is
removed from the wheel and wound on 54-inch (137 cm) diameter reels.
Emulsion
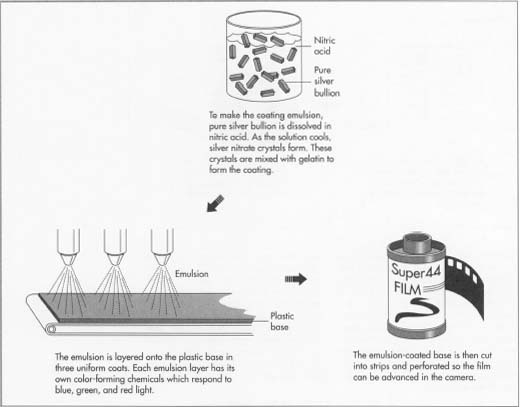
- 2 Silver is the main ingredient of the emulsion. Pure silver bullion is received at the manufacturing plant in bars that are checked by weight and serial number. The bars are dissolved in a strong solution of nitric acid, and the process releases heat. After the acid has completely dissolved the silver, the solution is stirred constantly and cooled. Cooling causes crystals of silver nitrate to grow, much like salt crystals in water. The crystals are wet with water that also separates out. The crystals are removed from the solution and whirled in centrifuges with sieve-like openings to remove the water and keep the crystals pure. At this point in the process, the chemical solutions are light-sensitive, so further manufacturing processes are completed in darkness.
- 3 Meanwhile, gelatin has been made using distilled water and treated with chemicals including potassium iodide and potassium bromide. The gelatin serves as a binding agent to hold the silver nitrate crystals, and also to fix them to the base. The gelatin and chemicals are mixed in cookers that are lined with silver so the emulsion remains pure. As the mixture cools, silver halide salts (chemical combinations of the silver, iodide, and bromide) form as fine crystals that remain suspended in the gelatin to make the emulsion.
Coating process
- 4 The emulsion is pumped through a piping system to "coating alley," a huge work area that may be 200 feet (61 m) wide and five stories high. The area must be immaculately clean and dust-free, and the operations of the roll-coating machines are controlled by arrays of control panels in the fully automated process. Machines coat precise amounts of emulsion in micro-thin layers on the wide strips of plastic base; a single, dried layer of emulsion may be six one-hundred-thousandths of an inch thick. Successive layers of three emulsions are applied to the base to make color film, and each emulsion layer has its own color-forming chemicals called linked dyes. The three emulsion layers in color film respond to blue, green, and red light, so each photograph is a triple latent image with the sandwiched color range reproduced by processing. The strips of emulsion-coated base (now film) are cut into progressively narrower widths, perforated so the film can be advanced in the camera, and spooled, except for instant film and sheet film that are packed flat.
Packaging
-
5 Film is packed in cartridges, cassettes, rolls, instant packs, or
sheets. Cartridges are used in certain types of cameras and include a
take-up spool that is built in so the exposed film and cartridge are
removed as a unit. Cassettes are made for cameras that use film in the
35-millimeter format. They consist of a spool enclosed in a metal
jacket. The tongue of the film is drawn over the pressure plate at the
back of the camera to a take-up spool that is built into the camera.
When the film is finished, it is rewound onto the spool in the cassette,
and the unit is removed. Rollfilms consist of paper-backed film that is
packed on a spool like the one in the camera. The film is wound onto the
spool in the camera, and that spool and film are removed. The spool on
which the film was packed originally can then be moved to the receiving
side of the camera, and a new roll inserted. The packs for instant
cameras contain 8 to 12 sheets that are ejected individually after each
shot. Sheet film is used for specialized applications like x-ray film.
Plastic cartridges for cartridge-type film are made by injection molding, in which fluid-like plastic is squirted mechanically into forms or molds. These are hardened, removed from the molds, and trimmed and smoothed. The spooled film is then placed in the cartridges and sealed. The metal canisters are printed on the outside, cut to shape and size, trimmed and smoothed, and edged with protective plastic. The metal is shaped around the spools of film. Plastic canisters and caps are also made for the film canisters, as are other types of outer packaging such as foil-lined paper pouches, and the outer cartons. The packaging is dated, shrink-wrapped in plastic in quantities appropriate for sale, packed in cardboard containers for shipping, and stored in air-conditioned rooms to await shipment.
Quality Control
In all phases of manufacture, photographic film is extremely sensitive to light, heat, dust, and impurities. Air flow into the film-manufacturing rooms is washed and filtered. Temperature and humidity are carefully regulated. Production rooms are scrubbed clean daily, and plant workers wear protective clothing and enter sensitive work areas through air showers that clean personnel of dust and contaminants. Each step of manufacture is carefully inspected and controlled. For example, the chromium-plated wheel on which the base is formed is inspected to maintain a mirror-like finish because tiny imperfections will affect the quality of the film. Finally, samples of film are removed from completed batches and subjected to many tests, including the taking of photographs with the samples.
Byproducts/Waste
Factory workers and the environment must also be protected from the hazardous chemicals, fumes, and wastes that can be generated during the process. Protective clothing keeps the product clean and insulates the workers from possible contaminants. Air released to the outside is also filtered and monitored. Extensive recycling is done, not only to protect the environment but also to salvage valuable materials such as silver for purifying and reuse. The photographic film industry was also among the first to use incineration successfully to burn wastes efficiently and control emissions.
The Future
Film manufacturers are continually improving the quality of film so that photographs are sharper, color is truer, graininess is reduced, and film speed is improved. Several new camera films use "T-grain" emulsion technology, in which the molecular structure of the silver halide crystals is modified to create silver grains shaped like tiny tablets. The flat shape helps them collect light efficiently, so sharper photographs are produced from higher-speed films. This technology also benefits the environment because fewer chemicals are needed for processing film, and the opportunity for chemicals to enter the environment is reduced.
The next advance in photography does not require film at all; the film-free camera stores photographs digitally without any film. Digital cameras electronically transfer images to computers which can then print the images.
Where To Learn More
Books
Bailey, Adrian and Adrian Holloway. The Book of Color Photography. Alfred A. Knopf, 1979.
Collins, Douglas. The Story of Kodak. Harry N. Abrams, Inc., Publishers, 1990.
Periodical
Antonoff, Michael. "Digital Snapshots from My Vacation." Popular Science, June 1995, pp. 72-76.
Other
From Glass Plates to Digital Images. Eastman Kodak Company, 1994. 343 State St., Rochester, NY 14650. (716)724-4000.
— Gillian S. Holmes
Comment about this article, ask questions, or add new information about this topic: