Copper
Background
Copper is one of the basic chemical elements. In its nearly pure state, copper is a reddish-orange metal known for its high thermal and electrical conductivity. It is commonly used to produce a wide variety of products, including electrical wire, cooking pots and pans, pipes and tubes, automobile radiators, and many others. Copper is also used as a pigment and preservative for paper, paint, textiles, and wood. It is combined with zinc to produce brass and with tin to produce bronze.
Copper was first used as early as 10,000 years ago. A copper pendant from about 8700 B.C. was found in what is now northern Iraq. There is evidence that by about 6400 B.C. copper was being melted and cast into objects in the area now known as Turkey. By 4500 B.C. , this technology was being practiced in Egypt as well. Most of the copper used before 4000 B.C. came from the random discovery of isolated outcroppings of native copper or from meteorites that had impacted Earth. The first mention of the systematic extraction of copper ore comes from about 3800 B.C. when an Egyptian reference describes mining operations on the Sinai Peninsula.
In about 3000 B.C. , large deposits of copper ore were found on the island of Cyprus in the Mediterranean Sea. When the Romans conquered Cyprus, they gave the metal the Latin name aes cyprium, which was often shortened to cyprium. Later this was corrupted to cuprum, from which the English word copper and the chemical symbol Cu are derived.
In South America, copper objects were being produced along the northern coast of Peru as early as 500 B.C. , and the development of copper metallurgy was well advanced by the time the Inca empire fell to the conquering Spanish soldiers in the 1500s.
In the United States, the first copper mine was opened in Branby, Connecticut, in 1705, followed by one in Lancaster, Pennsylvania, in 1732. Despite this early production, most copper used in the United States was imported from Chile until 1844, when mining of large deposits of high-grade copper ore around Lake Superior began. The development of more efficient processing techniques in the late-1800s allowed the mining of lower-grade copper ores from huge open-pit mines in the western United States.
Today, the United States and Chile are the world's top two copper producing countries, followed by Russia, Canada, and China.
Raw Materials
Pure copper is rarely found in nature, but is usually combined with other chemicals in the form of copper ores. There are about 15 copper ores mined commercially in 40 countries around the world. The most common are known as sulfide ores in which the copper is chemically bonded with sulfur. Others are known as oxide ores, carbonate ores, or mixed ores depending on the chemicals present. Many copper ores also contain significant quantities of gold, silver, nickel, and other valuable metals, as well as large quantities of commercially useless material. Most of the copper ores mined in the United States contain only about 1.2-1.6% copper by weight.
The most common sulfide ore is chalcopyrite, CuFeS 2 , also known as copper pyrite or yellow copper ore. Chalcocite, Cu 2 S, is another sulfide ore.
Cuprite, or red copper ore, Cu 2 O, is an oxide ore. Malachite, or green copper ore, Cu(OH) 2 •CuCO 3 , is an important carbonate ore, as is azurite, or blue copper carbonate, Cu(OH) 2 •2CuCO 3 .
Other ores include tennantite, boronite, chrysocolla, and atacamite.
In addition to the ores themselves, several other chemicals are often used to process and refine copper. These include sulfuric acid, oxygen, iron, silica, and various organic compounds, depending on the process used.
The Manufacturing
Process
The process of extracting copper from copper ore varies according to the type of ore and the desired purity of the final product. Each process consists of several steps in which unwanted materials are physically or chemically removed, and the concentration of copper is progressively increased. Some of these steps are conducted at the mine site itself, while others may be conducted at separate facilities.
Here are the steps used to process the sulfide ores commonly found in the western United States.
Mining
- 1 Most sulfide ores are taken from huge open-pit mines by drilling and blasting with explosives. In this type of mining, the material located above the ore, called the overburden, is first removed to expose the buried ore deposit. This produces an open pit that may grow to be a mile or more across. A road to allow access for equipment spirals down the interior slopes of the pit.
- 2 The exposed ore is scooped up by large power shovels capable of loading 500-900 cubic feet (15-25 cubic meters) in a single bite. The ore is loaded into giant dump trucks, called haul trucks, and is transported up and out of the pit.
Concentrating
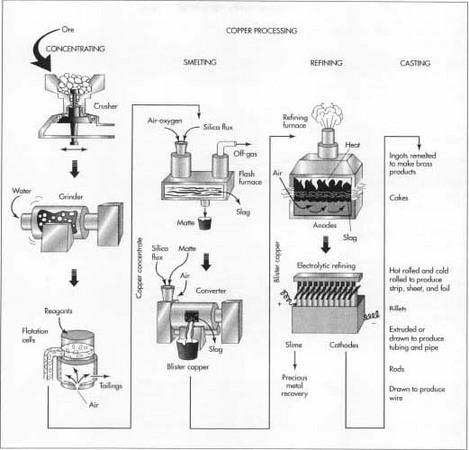
The copper ore usually contains a large amount of dirt, clay, and a variety of non-copper bearing minerals. The first step is to remove some of this waste material. This process is called concentrating and is usually done by the flotation method.
- 3 The ore is crushed in a series of cone crushers. A cone crusher consists of an interior grinding cone that rotates on an eccentric vertical axis inside a fixed outer cone. As the ore is fed into the top of the crusher, it is squeezed between the two cones and broken into smaller pieces.
- 4 The crushed ore is then ground even smaller by a series of mills. First, it is mixed with water and placed in a rod mill, which consists of a large cylindrical container filled with numerous short lengths of steel rod. As the cylinder rotates on its horizontal axis, the steel rods tumble and break up the ore into pieces about 0.13 in (3 mm) in diameter. The mixture of ore and water is further broken up in two ball mills, which are like a rod mill except steel balls are used instead of rods. The slurry of finely ground ore that emerges from the final ball mill contains particles about 0.01 in (0.25 mm) in diameter.
- 5 The slurry is mixed with various chemical reagents, which coat the copper particles. A liquid, called a frother, is also added. Pine oil or long-chain alcohol are often used as frothers. This mixture is pumped into rectangular tanks, called flotation cells, where air is injected into the slurry through the bottom of the tanks. The chemical reagents make the copper particles cling to the bubbles as they rise to the surface. The frother forms a thick layer of bubbles, which overflows the tanks and is collected in troughs. The bubbles are allowed to condense and the water is drained off. The resulting mixture, called a copper concentrate, contains about 25-35% copper along with various sulfides of copper and iron, plus smaller concentrations of gold, silver, and other materials. The remaining materials in the tank are called the gangue or tailings. They are pumped into settling ponds and allowed to dry.
Smelting
Once the waste materials have been physically removed from the ore, the remaining copper concentrate must undergo several chemical reactions to remove the iron and sulfur. This process is called smelting and traditionally involves two furnaces as described below. Some modern plants utilize a single furnace, which combines both operations.
- 6 The copper concentrate is fed into a furnace along with a silica material, called a flux. Most copper smelters utilize oxygen-enriched flash furnaces in which preheated, oxygen-enriched air is forced into the furnace to combust with fuel oil. The copper concentrate and flux melt, and collect in the bottom of the furnace. Much of the iron in the concentrate chemically combines with the flux to form a slag, which is skimmed off the surface of the molten material. Much of the sulfur in the concentrate combines with the oxygen to form sulfur dioxide, which is exhausted from the furnace as a gas and is further treated in an acid plant to produce sulfuric acid. The remaining molten material in the bottom of the furnace is called the matte. It is a mixture of copper sulfides and iron sulfides and contains about 60% copper by weight.
- 7 The molten matte is drawn from the furnace and poured into a second furnace called a converter. Additional silica flux is added and oxygen is blown through the molten material. The chemical reactions in the converter are similar to those in the flash furnace. The silica flux reacts with the remaining iron to form a slag, and the oxygen reacts with the remaining sulfur to form sulfur dioxide. The slag may be fed back into the flash furnace to act as a flux, and the sulfur dioxide is processed through the acid plant. After the slag is removed, a final injection of oxygen removes all but a trace of sulfur. The resulting molten material is called the blister and contains about 99% copper by weight.
Refining
Even though copper blister is 99% pure copper, it still contains high enough levels of sulfur, oxygen, and other impurities to hamper further refining. To remove or adjust the levels of these materials, the blister copper is first fire refined before it is sent to the final electrorefining process.
- 8 The blister copper is heated in a refining furnace, which is similar to a converter described above. Air is blown into the molten blister to oxidize some impurities. A sodium carbonate flux may be added to remove traces of arsenic and antimony. A sample of the molten material is drawn and an experienced operator determines when the impurities have reached an acceptable level. The molten copper, which is about 99.5% pure, is then poured into molds to form large electrical anodes, which act as the positive terminals for the electrorefining process.
- 9 Each copper anode is placed in an individual tank, or cell, made of polymer-concrete. There may be as many as 1,250 tanks in operation at one time. A sheet of copper is placed on the opposite end of the tank to act as the cathode, or negative terminal. The tanks are filled with an acidic copper sulfate solution, which acts as an electrical conductor between the anode and cathode. When an electrical current is passed through each tank, the copper is stripped off the anode and is deposited on the cathode. Most of the remaining impurities fall out of the copper sulfate solution and form a slime at the bottom of the tank. After about 9-15 days, the current is turned off and the cathodes are removed. The cathodes now weigh about 300 lb (136 kg) and are 99.95-99.99% pure copper.
- 10 The slime that collects at the bottom of the tank contains gold, silver, selenium, and tellurium. It is collected and processed to recover these precious metals.
Casting
- 11 After refining, the copper cathodes are melted and cast into ingots, cakes, billets, or rods depending on the final application. Ingots are rectangular or trapezoidal bricks, which are remelted along with other metals to make brass and bronze products. Cakes are rectangular slabs about 8 in (20 cm) thick and up to 28 ft (8.5 m) long. They are rolled to make copper plate, strip, sheet, and foil products. Billets are cylindrical logs about 8 in (20 cm) in diameter and several feet (meters) long. They are extruded or drawn to make copper tubing and pipe. Rods have a round cross-section about 0.5 in (1.3 cm) in diameter. They are usually cast into very long lengths, which are coiled. This coiled material is then drawn down further to make copper wire.
Quality Control
Because electrical applications require a very low level of impurities, copper is one of the few common metals that are refined to almost 100% purity. The process described above has been proven to produce copper of very high purity. To ensure this purity, samples are analyzed at various steps to determine whether any adjustment to the process is required.
Byproducts/Waste
The recovery of sulfuric acid from the copper smelting process not only provides a profitable byproduct, but also significantly reduces the air pollution caused by the furnace exhaust. Gold, silver, and other precious metals are also important byproducts.
Waste products include the overburden from the mining operation, the tailings from the concentrating operation, and the slag from the smelting operation. This waste may contain significant concentrations of arsenic, lead, and other chemicals, which pose a potential health hazard to the surrounding area. In the United States, the Environmental Protection Agency (EPA) regulates the storage of such wastes and the remediation of the area once mining and processing operations have ceased. The sheer volume of the material involved—in some cases, billions of tons of waste—makes this a formidable task, but it also presents some potentially profitable opportunities to recover the useable materials contained in this waste.
The Future
Demand for copper is expected to remain high, especially in the electrical and electronics industries. The current trends in copper processing are towards methods and equipment that use less energy and produce less air pollution and solid waste. In the United States, this is a difficult assignment because of the stringent environmental controls and the very low-concentration copper ores that are available. In some cases, the production costs may increase significantly.
One encouraging trend is the increased use of recycled copper. Currently over half the copper being produced in the United States comes from recycled copper. Fifty-five percent of the recycled copper comes from copper machining operations, such as screw forming, and 45% comes from the recovery of used copper products, such as electrical wire and automobile radiators. The percentage of recycled copper is expected to grow as the costs of new copper processing increase.
Where to Learn More
Books
Brady, George S., Henry R. Clauser, and John A. Vaccari. Materials Handbook. McGraw-Hill, 1997.
Heiserman, David L. Exploring Chemical Elements and Their Compounds. TAB Books, 1992.
Hombostel, Caleb. Construction Materials. John Wiley and Sons, Inc., 1991.
Kroschwitz, Jacqueline I. and Mary Howe-Grant, ed. Encyclopedia of Chemical Technology. John Wiley and Sons, Inc., 1993.
Stwertka, Albert. A Guide to the Elements. Oxford University Press, 1996.
Periodicals
Baum, Dan and Margaret L. Knox. "We want people who have a problem with mine wastes to think of Butte." Smithsonian (November 1992): 46-52, 54-57.
Shimada, Izumi and John F. Merkel. "Copper-Alloy Metallurgy in Ancient Peru." Scientific American (July 1991): 80-86.
Other
— Chris Cavette
I WANT TO MAKE COPPER SULFATE